Call 1-877-355-4447
Monday–Friday
8 AM to 8 PM ET
Hizentra ConnectSM provides the resources healthcare providers and patients need for consistent access to immune globulin (Ig) therapies from CSL Behring. Discover all of the ways we can help.
With proper training, your patients can self-administer Hizentra—so you can plan treatment to fit their lifestyle.
See how easy self-administration can be, and review administration options. Plus, find downloadable resources and step-by-step videos to help your patients.

We offer highly effective tools to help patients start and stay on Hizentra. Talk to your Hizentra representative to learn more or to order materials.
The kit provides resources to help patients reach their goals and take control of their Ig treatment.
This Hizentra kit for children includes the items in the adult kit plus:
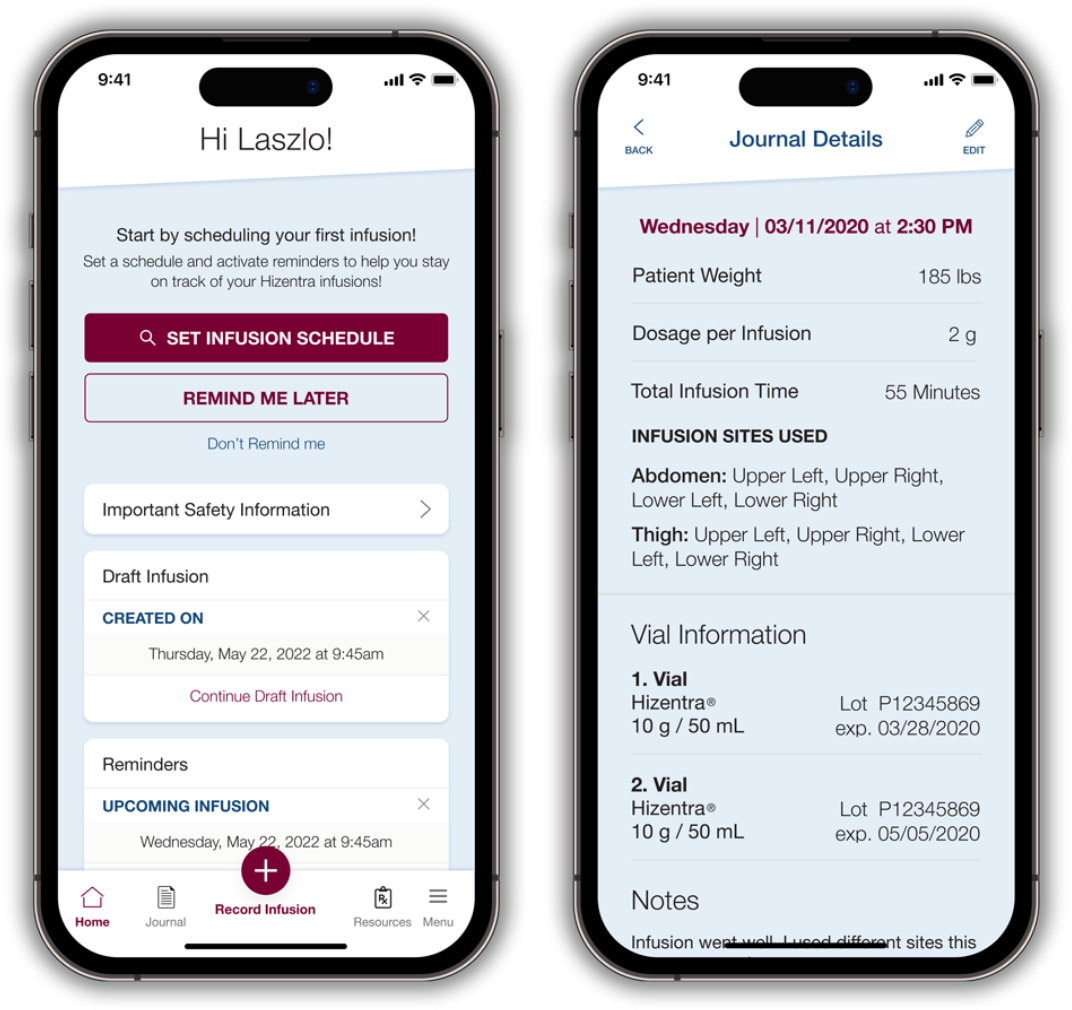
The Hizentra app lets your patients:
The app provides a clearer understanding of your patients’ infusion history, which can help you adjust treatment as necessary.
Existing MyHizentra® Infusion Manager app users will automatically transition to the Hizentra app when they update their app.
Apple, the Apple logo, iPad, and iPhone are trademarks of Apple Inc., registered in the US and other countries. App Store is a service mark of Apple Inc. By clicking on the App Store link above, you will be taken to a website hosted by Apple.
Android and Google Play are trademarks of Google Inc. By clicking on the Google Play link above, you will be taken to a site hosted by Google Inc.
To help you find nurses in your area who are experts in teaching patients how to self-infuse Hizentra, we offer an online locator of nurses who have completed our SHARE Nurse Training.
Become an expert and optimize patient care with nurse education and training on SCIg administration with Hizentra.
Help patients learn about PI or CIDP and how you can work together to plan when and where they infuse around their life, instead of planning life around treatments. Order the Hizentra brochure for PI or CIDP.

These programs include live events and webinars—featuring a presentation from a nurse educator who treats patients who use Hizentra.
Find in-person events and webinars for your patients